A solar panel made of bacteria
"Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis"
Science Vol. 352, Issue 6290, pp. 1210-1213
Science Vol. 352, Issue 6290, pp. 1210-1213
"Among renewable energy resources, solar energy is by far the largest exploitable resource, providing
more energy in 1 hour to the earth than all of the energy consumed by humans in an entire year."
Nathan Lewis & Daniel Nocera, PNAS
Sun gods: Splitting a water molecule
The sun is the source of most of the energy that powers life on Earth. Through the process of photosynthesis - the chemical reaction used by plants to generate sugar from carbon dioxide, water and sunlight - the energy from the sun is converted into biological life.
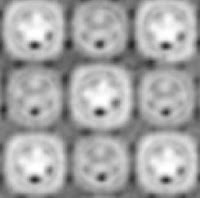 |
| Electron microscope image of crystals of the photosynthesis reaction center |
The leaves of plants; seaweed; algae; moss; tiny blue-green bacteria - all these things have a greenish tint because they contain the molecule chlorophyll, a green colored organic molecule.
Chlorophyll absorbs energy from the rays of the sun, and directs this energy to an intricate molecular structure called the photosynthetic reaction center, made of several different proteins and other molecules.
Each photosynthetic reaction center is a tiny, minuscule thing - much smaller than a single cell, which can contain hundreds of individual reaction centers.
Yet despite their minuscule size, something remarkable and miraculous occurs inside them. Inside the reaction center, the energy absorbed from sunlight is used to split water molecules into hydrogen and oxygen.
The chemical bonds in a water molecule are extremely strong compared to the bonds in organic molecules like sugars and proteins. Using sunlight to break these bonds releases a lot of energy, which the cell is able to store as chemical energy in the form of the molecules ATP and NADPH.
All organisms throughout the kingdoms of life use these two molecules as a universal store of energy - ATP molecules are sometimes called the 'energy currency' of the cell.
The plants then use the ATP and NADPH generated by splitting water molecules to convert carbon dioxide to sugar in a complex series of biochemical reactions known as the light-independent reactions of photosynthesis (also known as the Calvin Cycle).
The sugar created this way provides the material for the plant to grow and reproduce. Animals and other organisms feed on the plants. Other animals eat the plant eaters, and when all these things die bacteria and fungi feed on their corpses. But all of the energy and matter that makes this entire cycle has a single source - sugar produced by plants from sunlight, water and air.
In this way, the Sun makes nearly all life on Earth possible. Most ancient cultures have a central role for sun gods, because they understood that in some mysterious way this big ball of fire in the sky made it possible for their crops to grow. It is only through the insights of very recent science that we have begun to probe the nanoscale structures of the reaction centers that make this miracle possible.
Photosynthesis is the source for all human food, and thus for the energy that we have always relied on to sustain ourselves. For most of human history, agriculture has been the engine that drove human societies and civilizations.
But the human capacity for invention eventually allowed our species to tap into much larger reservoirs of energy.
Buried sunshine: The fossil fuel revolution
Buried sunshine: The fossil fuel revolution
The technologies of industrialization allowed us to burn fossil fuels like coal and oil to generate motion for transport and large-scale industrial automation. The invention of electricity allowed us to transmit and utilize this energy in completely novel ways, leading to light-bulbs, electronic appliances and the entire computing revolution.
From the earliest days of industrialization, we have understood the utility of digging deep in the ground to extract oil and coal. We learnt that these are incredibly dense stores of energy, and we utilized them on a vast scale to create modern, industrialized human societies.
 |
| Fossil fuels store ancient photosynthetic energy |
Where does the energy inside these fossil fuels come from? Fossil fuels are made from the decomposition and fossilization of ancient plants and animals that died millions of years ago. The bodies of these organisms contain chemical energy in the form of the chemical bonds in their sugars, fats and proteins. These chemical bonds were able to exist because ancient plants converted air, water and sunlight into sugar.
Fossil fuels store the energy from ancient water-splitting reactions in the form of coal, oil and natural gas. When we dig them up, we can burn them to release this energy for our own use - obtaining far more energy than would be possible from all the living plants in the world.
We can tap into the stored energy of thousands of years of combined photosynthesis when we extract and burn fossil fuels. The amount of energy contained in these deposits vastly exceeds that which can be produced by living plants, because the dead always outnumber the living.
To this day, fossil fuels account for the vast majority of the energy produced by human beings. But this is becoming a problem. Fossil fuels have been stored in the ground for millions of years, but humans are now burning them on a large scale in a very short period of time. This is resulting in much greater amounts of carbon dioxide being released in the air, and is the cause of the ongoing and potentially catastrophic problems of climate change and global warming.
There is a pressing need, therefore, to find carbon-neutral energy sources.
Carbon-neutral energy sources do not increase the amount of carbon dioxide on the planet when they produce power. One example is nuclear power - nuclear power does not rely on carbon atoms at any point to generate energy, relying instead in the process of nuclear fission in heavy radioactive metals like uranium. Thus, nuclear power will never generate carbon dioxide and contribute to global warming. However it has severe shortcomings - the storage of nuclear waste and the risk of a devastating nuclear meltdown high among them.
Renewable energy sources like wind and solar are currently too expensive and inefficient to become a realistic alternative to fossil fuels, but there are many well-funded and plausible research projects that could make cost-effective renewable energy a reality in the recent future.
In solar power generation, solar panels constructed of special materials are exposed to sunlight to generate energy. This can be done in a number of ways. In photovoltaic panels, solar energy is converted directly into electric current using special semiconducting materials. In solar thermal collection, sunlight is converted to heat, which can be used directly or turned into electricity.
However, both these forms of solar power generation suffer from a severe shortcoming - there is no easy way to store the energy generated by them. Because the planet has day/night cycles, it is difficult for these forms of solar power to generate all our energy needs because they would only work during daylight hours and clear skies.
Photosynthesis does find a way to easily store the energy from sunlight - it stores it in the form of chemical bonds in sugar and other organic molecules. This form of storage is incredibly stable and effective - just look at the fossil fuels that provide most of our energy needs
This biological example has inspired some scientists to try and create systems for artificial photosynthesis.
Daniel Nocera is a chemist whose group has been trying to make inorganic catalysts that can mimic the organic reaction centers that split water molecules in photosynthesis. Whereas plants use a complex of proteins and other organic molecules to use sunlight to split water into hydrogen and oxygen, Nocera's group is trying to create inorganic complexes of metals that can carry out the same reaction.
An artificial leaf
In a breakthrough study in 2008, the group discovered a cheap catalyst made of cobalt and phosphate that could catalyze the water splitting reaction, analogous to the reaction center in photosynthesis.
Just as the reaction center in a leaf cannot capture solar energy without chlorophyll, the cobalt catalyst requires a separate component to do this. The group went on to combine this water splitting cobalt catalyst with a silicon semiconductor that mimics the action of chlorophyll, creating an 'artifical leaf'.
This artificial leaf takes sunlight and water, and produces hydrogen and oxygen. The hydrogen produced can be burnt to produce energy. Thus, this artificial leaf allows for the direct conversion of water and sunlight into a usable fuel - hydrogen.
A solar panel made of bacteria
In order for hydrogen to be used widely as a fuel, we would have to invest a vast amount of money developing the infrastructure to store and transport hydrogen in order to create a functioning hydrogen economy.
Without a working hydrogen economy, the artificial leaf created by Nocera's team cannot be a viable source of carbon-neutral energy.
Nocera's team overcame this problem by combining the artificial leaf with a living bacteria that can extract energy from hydrogen, and use this energy to create organic chemicals, including fuels like petrol.
Nocera collaborated with noted synthetic biologist Pamela Silver to create the hybrid device. They chose to use the bacteria Ralstonia eutropha. R. eutropha is a lithotroph - unlike most organisms, lithotrophs are able to extract energy from inorganic chemicals like minerals and rocks.
R. eutropha has the rare ability to extract energy from hydrogen and use this energy to convert carbon dioxide into sugars and other organic chemicals. Although wild R. eutropha does not naturally produce hydrocarbon fuels, it can be genetically engineered to synthesize these chemicals in large amounts.
By combining the artificial leaf with genetically engineered bacteria, the team were able to create a single device that could transform solar energy into easily usable organic fuels.
But their initial efforts had a drawback - the metals used generate toxic compounds called reactive oxygen species during the water splitting reaction, and these begin to kill the bacteria after some time.
In their most recent paper, the collaborative groups overcame this hurdle by developing a new artificial leaf for the water splitting reaction.
They replaced some of the metals used with a new cobalt-phosphorus alloy that does not produce toxic reactive oxygen species, creating a system that supports bacterial life and is 'bio-compatible'.
The new artificial leaf is 'bio-compatible', allowing for full integration with biological components like genetically engineered bacteria. This bio-compatible, hybrid system is able to take in sunlight, air and water, and convert it directly to carbon-neutral fuels.
Although it will take many years for this breakthrough technology to be commercialized, it has the potential to one day provide a source of cheap, renewable energy and completely transform human society.
Renewable energy sources like wind and solar are currently too expensive and inefficient to become a realistic alternative to fossil fuels, but there are many well-funded and plausible research projects that could make cost-effective renewable energy a reality in the recent future.
 |
| A solar tower |
However, both these forms of solar power generation suffer from a severe shortcoming - there is no easy way to store the energy generated by them. Because the planet has day/night cycles, it is difficult for these forms of solar power to generate all our energy needs because they would only work during daylight hours and clear skies.
Photosynthesis does find a way to easily store the energy from sunlight - it stores it in the form of chemical bonds in sugar and other organic molecules. This form of storage is incredibly stable and effective - just look at the fossil fuels that provide most of our energy needs
This biological example has inspired some scientists to try and create systems for artificial photosynthesis.
Daniel Nocera is a chemist whose group has been trying to make inorganic catalysts that can mimic the organic reaction centers that split water molecules in photosynthesis. Whereas plants use a complex of proteins and other organic molecules to use sunlight to split water into hydrogen and oxygen, Nocera's group is trying to create inorganic complexes of metals that can carry out the same reaction.
An artificial leaf
 |
Schematic diagram of the artificial leaf:
Co-OEC - water splitting cobalt catalyst;
3jn-a-Si - silicon absorbs sunlight energy';
|
Just as the reaction center in a leaf cannot capture solar energy without chlorophyll, the cobalt catalyst requires a separate component to do this. The group went on to combine this water splitting cobalt catalyst with a silicon semiconductor that mimics the action of chlorophyll, creating an 'artifical leaf'.
This artificial leaf takes sunlight and water, and produces hydrogen and oxygen. The hydrogen produced can be burnt to produce energy. Thus, this artificial leaf allows for the direct conversion of water and sunlight into a usable fuel - hydrogen.
A solar panel made of bacteria
 |
The artificial leaf is combined with living bacteria - R. eutropha can extract energy from hydrogen and use this to produce chemicals like petrol (source) |
Without a working hydrogen economy, the artificial leaf created by Nocera's team cannot be a viable source of carbon-neutral energy.
Nocera's team overcame this problem by combining the artificial leaf with a living bacteria that can extract energy from hydrogen, and use this energy to create organic chemicals, including fuels like petrol.
Nocera collaborated with noted synthetic biologist Pamela Silver to create the hybrid device. They chose to use the bacteria Ralstonia eutropha. R. eutropha is a lithotroph - unlike most organisms, lithotrophs are able to extract energy from inorganic chemicals like minerals and rocks.
R. eutropha has the rare ability to extract energy from hydrogen and use this energy to convert carbon dioxide into sugars and other organic chemicals. Although wild R. eutropha does not naturally produce hydrocarbon fuels, it can be genetically engineered to synthesize these chemicals in large amounts.
By combining the artificial leaf with genetically engineered bacteria, the team were able to create a single device that could transform solar energy into easily usable organic fuels.
But their initial efforts had a drawback - the metals used generate toxic compounds called reactive oxygen species during the water splitting reaction, and these begin to kill the bacteria after some time.
 |
| The new biocompatible artificial leaf only uses cobalt and phosphorus as catalysts (source) |
In their most recent paper, the collaborative groups overcame this hurdle by developing a new artificial leaf for the water splitting reaction.
They replaced some of the metals used with a new cobalt-phosphorus alloy that does not produce toxic reactive oxygen species, creating a system that supports bacterial life and is 'bio-compatible'.
The new artificial leaf is 'bio-compatible', allowing for full integration with biological components like genetically engineered bacteria. This bio-compatible, hybrid system is able to take in sunlight, air and water, and convert it directly to carbon-neutral fuels.
Although it will take many years for this breakthrough technology to be commercialized, it has the potential to one day provide a source of cheap, renewable energy and completely transform human society.
No comments:
Post a Comment